Which Measurement Is Directly Related To The Ph Of The Body
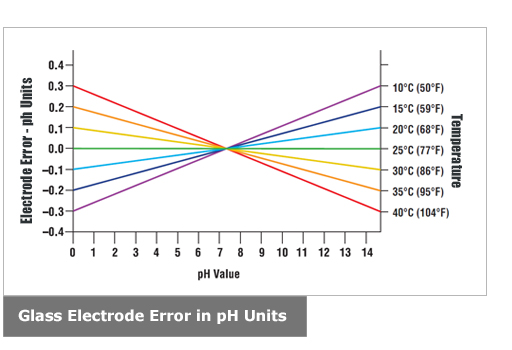
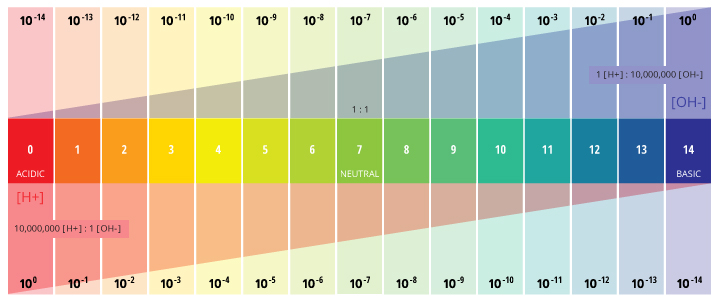
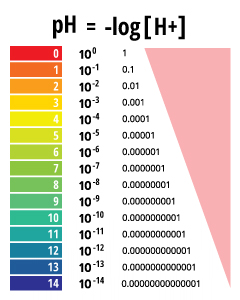
The voltage between the electrodes is directly proportional to the ph of the test solution. For the scientifically inclined ph or power of hydrogen is a measurement of the hydrogen ion concentration in your body. The alkalinity of water or a solution is the quantitative capacity of that solution to buffer or neutralize an acid. While alkalinity and ph are closely related there are distinct differences. Ph meter measures acid and base in a fluid. The proportionality constant depends on temperature so a temperature sensor is also necessary.
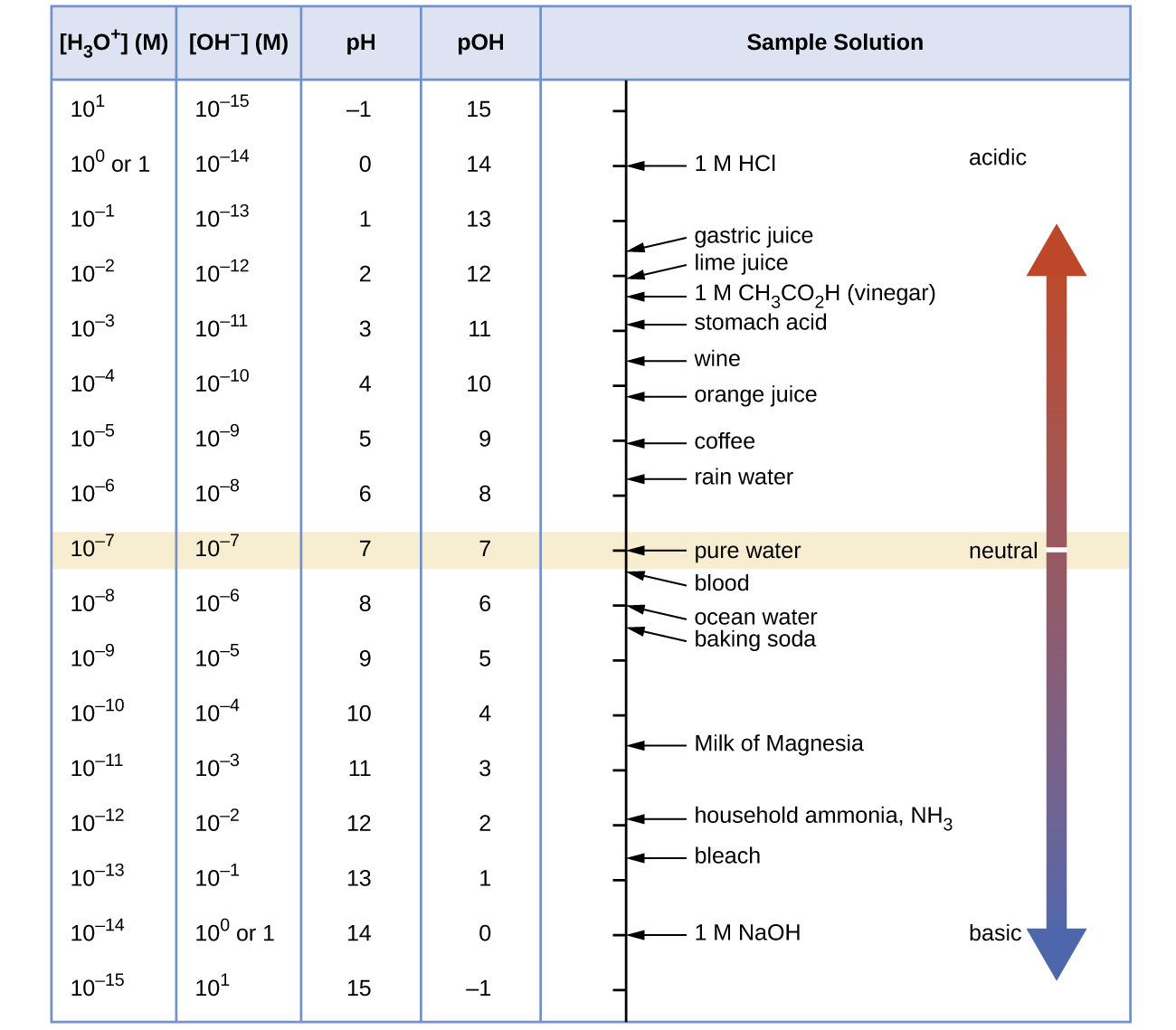
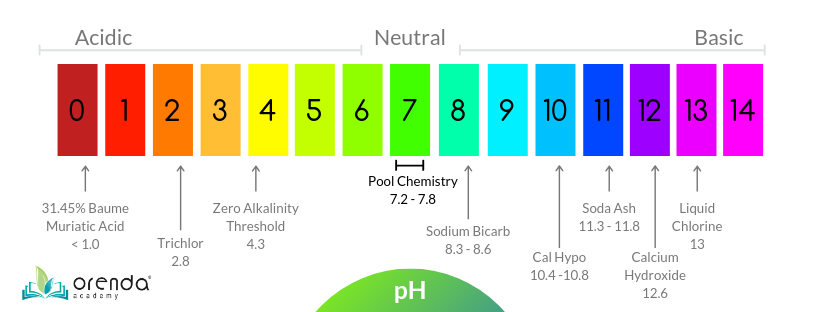
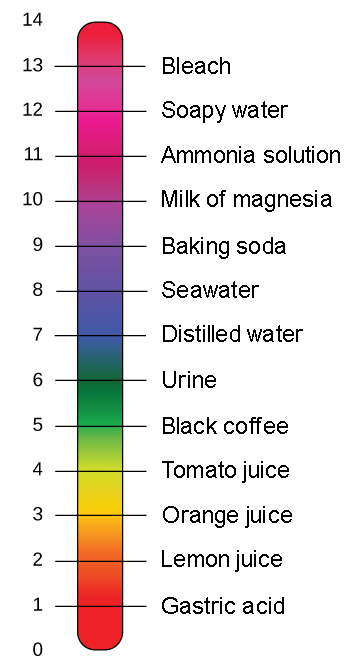
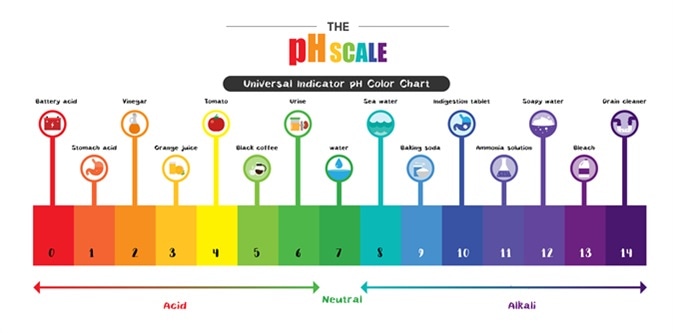
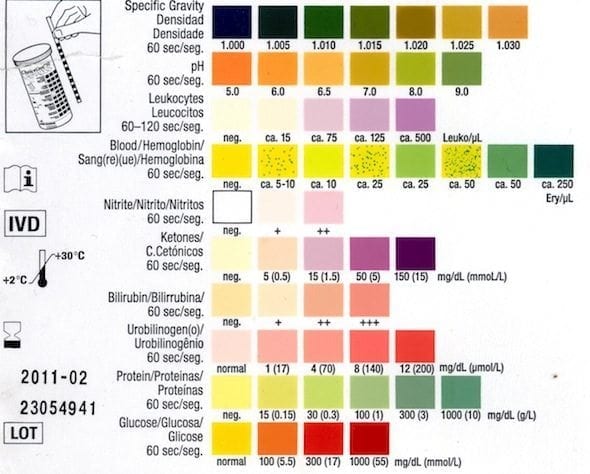
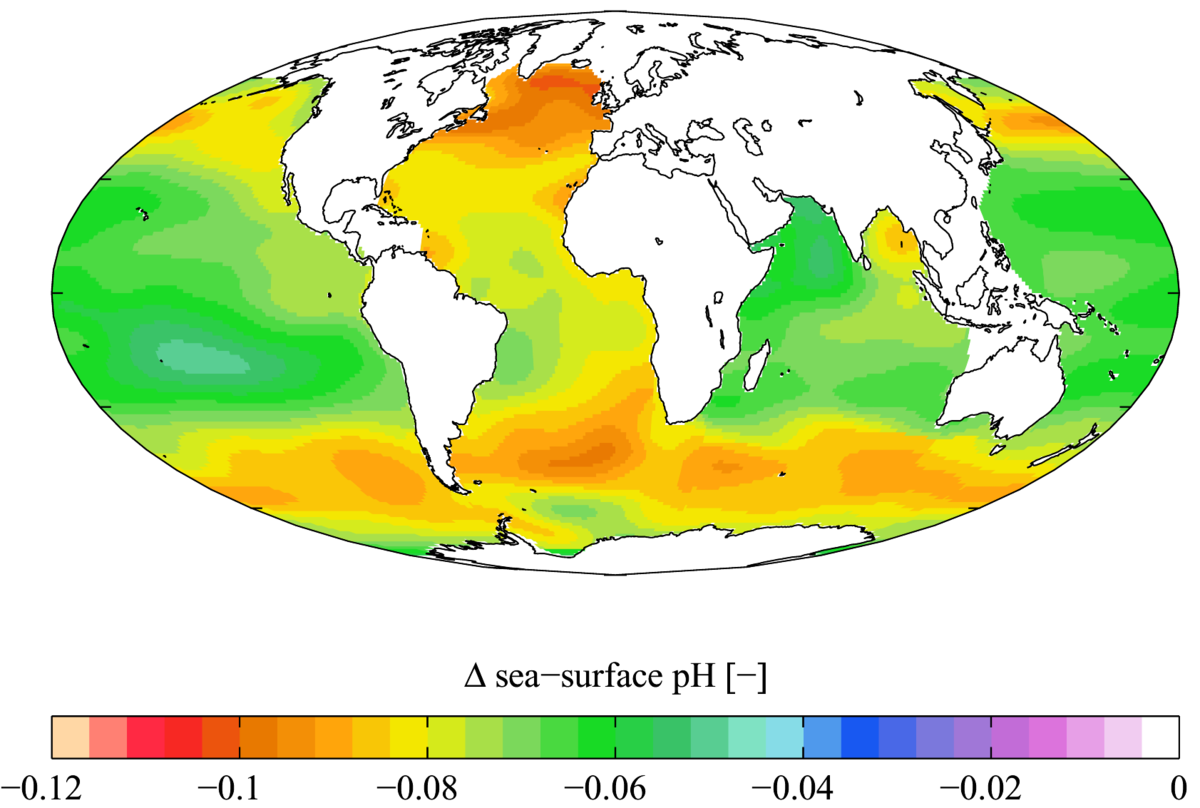
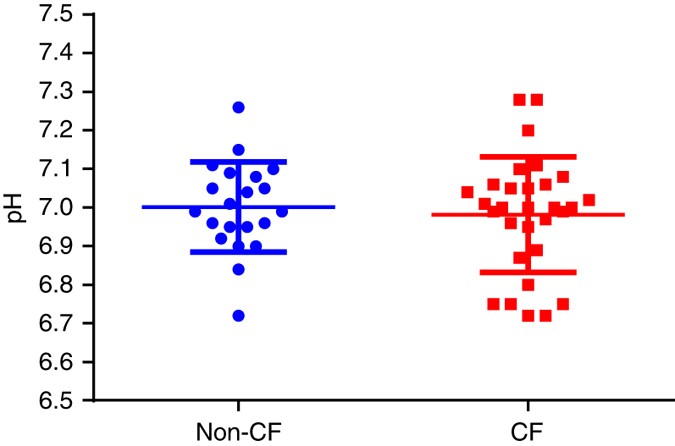
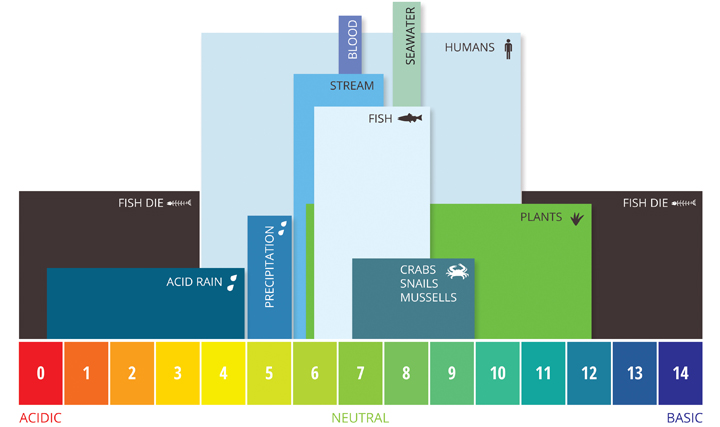
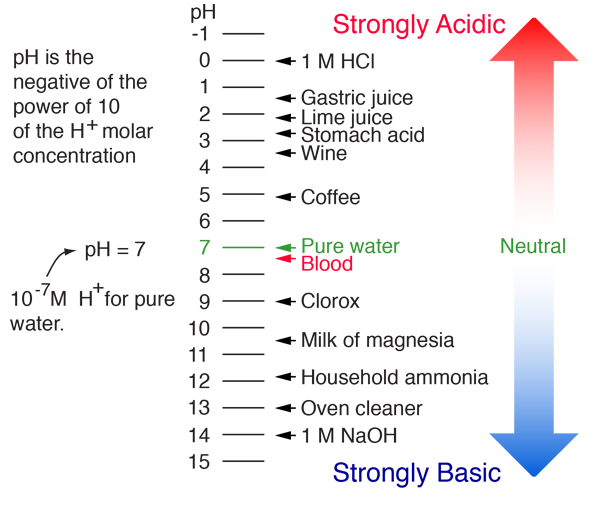
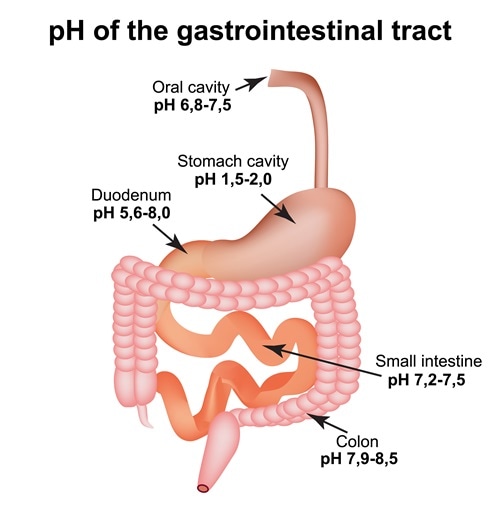
The parts and fluids of the human body have ph values. While 70 is considered neutral a score below 7 shows that your body is acidic while a score above indicates you have reduced the acidity to a healthy level. Your ph score represents the amount of acid in your body. When the solution is neutral it has a ph value of 7 when lesser than 7 it is acidic and more than 7 indicates it is basic solution. Nakao in encyclopedia of food microbiology second edition 2014. Two dimensional ph measurements of electrolyte were carried out at the bottom of an electrolysis cell.
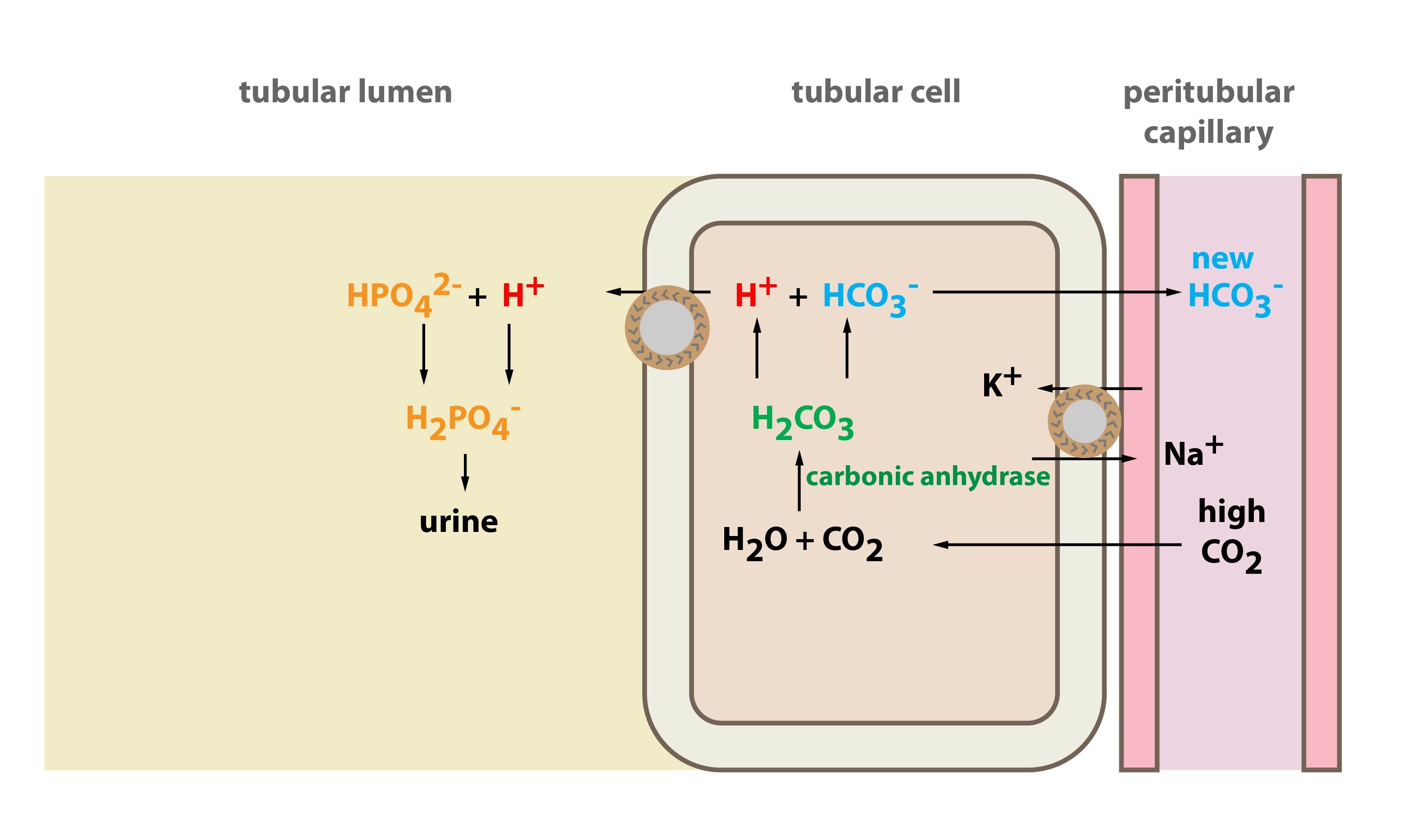
Alkalinity does not refer to alkalis as alkaline does ⁶. The cell consists of a measuring and reference electrode. Measurement of ph and related terminology. The chemical balance in a human body is determined due to the ph of blood and other fluids. It is only for very dilute solutions that the ph can be directly. The measured area included the points directly under both the anode and the cathode.
Alkalinity and ph are directly related at 100 air saturation. Hence ph is defined as hydrogen ion concentration in fluid. The measured area was 96 50 mm and the number of measuring points was 48 25.
Random Post
- tapasya nayak srivastava body measurement
- magic fox body measurements
- body circumference measurements body fat
- get body measurements iphone
- christina hendricks body measurement
- angelica maria body measurements
- best body measurements for girl
- blouse body measurement in kannada
- gnc body fat measurement
- jojo siwa body measurement
- weighing machine with body fat measurement
- rosalia body measurement
- square body measurement
- printable body measurement chart pdf
- siddharth sharma body measurement
- kim taehyung body measurements
- body structure measurement
- strapless bra measurement
- chloe bennet body measurement
- body measurement equipment
- dolph ziggler body measurement
- susan li body measurement
- body measurement body
- body part measurement facts
- zoe mclellan body measurement
- body measurement eeg
- ashnoor kaur body measurement
- jodie foster body measurements
- viola davis body measurement
- bra fitting jcpenney
- what's my body type measurements
- bts body measurements
- pak body measurement
- body measurement of children's wear
- padma lakshmi body measurement
- introduction of body measurements
- mel b body measurement
- rhea chakraborty body measurement
- maryam nawaz body measurement
- mtv body measurement
- maxene magalona body measurement
- marvelous mrs maisel body measurements scene
- devon windsor body measurement
- chitrangada singh body measurement
- sidhant gupta body measurement
- supermodel body measurement
- where to take body measurement
- body measurement ffxiv
- jisoo body measurement
- paz body measurement